Thin film deposition of metal oxides
Liquid phase deposition (LPD) is an aqueous soft chemistry technique for deposition of highly uniform metal oxide thin films. LPD was invented by Nagayama et al. for SiO2 films, and it was subsequently developed for other metal oxides/metal oxy-hydroxides thin films such as TiO2 SnO2, ZrO2, WO3, Nb2O5, MoO3, V2O5, NiO, Fe2O3, FeOOH, and NiOOH. This technique also has been developed for binary metal oxides such as spinel ferrites, hexaferrites, and perovskite titanates. LPD is based on the slow hydrolysis of metal-fluoro complex species at temperatures below 80°C. The hydrolysis occurs via an equilibrium reaction whereby OH gradually replaces the fluoride ions- ions and/or water molecules in a supersaturated solution, thereby yielding mixtures of metal hydroxides/oxyhydroxides. The desired metal oxides are obtained upon heat treatment at 400- 800 °C. As a simple and easily scalable deposition technique, LPD allows the deposition of metal oxide films on substrates with complex morphologies, and does not require expensive vacuum equipment as in the case of most physical deposition techniques.
Moreover, these reactions can be performed at low temperatures and are environmentally friendly. In the absence of a fluorine scavenger, the hydrolysis reaction is slow, and the equilibrium is reached. (Reaction 1). According to reaction 2, adding boric acid to the treatment solution will lead to the consumption of the fluoride ions with the formation of [BF4]- a stable water-soluble complex and allows the elimination of the F- ions from the resulting oxide.
(1)
(2)
Our group has successfully developed the LPD for the thin deposition of FePO4.2H2O and WO3.2H2O thin films.

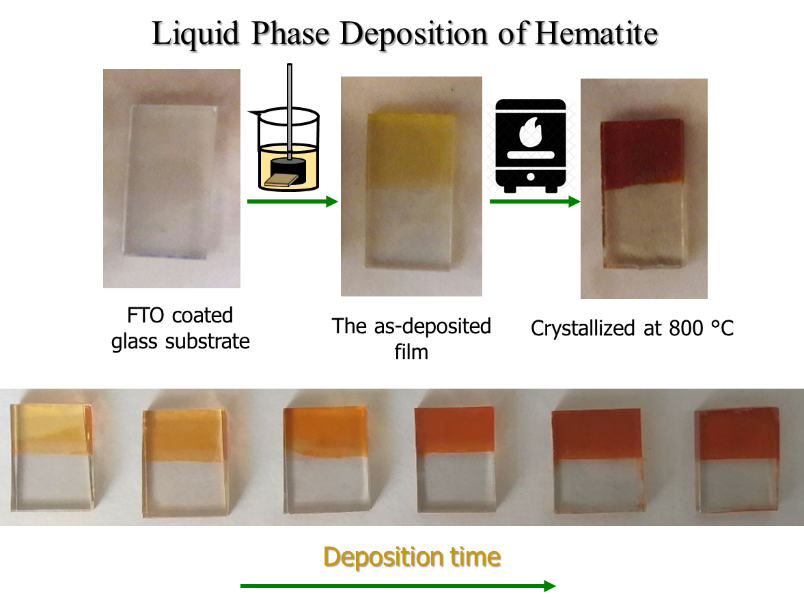

LPD for the hematite thin films, simply by varying the deposition time, the films with different thicknesses could be grown, and the FE-SEM image for a hematite thin film shows the film consists of densely packed small grains.
FePO4.2H2O thin films have potential application in Li-ion batteries, and WO3.2H2O shows promising electrochromic properties for applications in smart windows and displays. In the EC lab, the LPD process has been modified in such a way to deposit films with higher quality and better properties for the relevant applications.
Click here and here to see the performance of our EC films.
- Nagayama, H.; Honda, H.; Kawahara, H., A New Process for Silica Coating. J. Electrochem. Soc. 135 (1988) 2013-2016.
- Amin Yourdkhani, Daniela Caruntu, Armando K. Perez and Gabriel Caruntu, Liquid Phase Deposition of Barium Hexaferrite Thin Films, Journal of Physical Chemistry C,118 (2014) 1774–1782.
- Ehsan Mahboubi, Amin Yourdkhani and Reza Pourselehi, Liquid phase deposition of iron phosphate thin films, CrystEngComm, 20 (2018) 5256-5268.
- Maryam Mohammad-Hosseinpour, Amin Yourdkhani, Reza Poursalehi, Fast-switching electrochromic response of WO3·2H2O of plate-like particles synthesized by liquid phase deposition, Journal of Alloys and Compounds, 879 (2021) 160418-160426.
Photoelectrochemical water splitting of metal oxide thin films
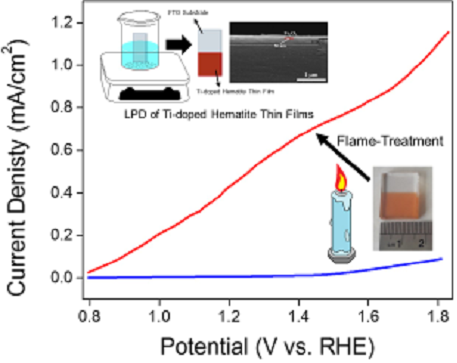
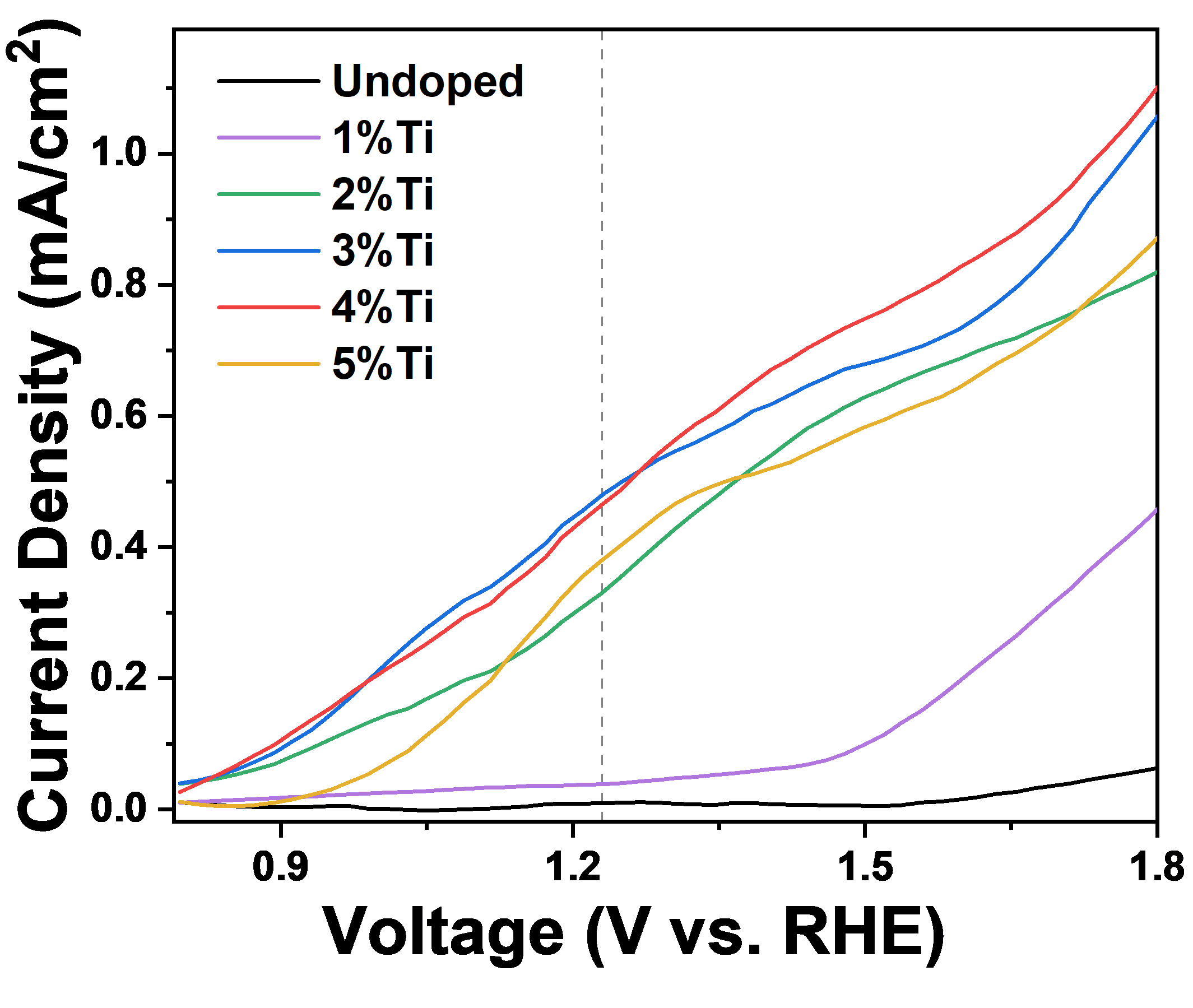
The alternative energies for fossil energies with zero impact on the environment is now essential for the world. Hydrogen is known as the cleanest fuel which upon combustion converts to water. One way to produce hydrogen is based on utilizing semiconductors as photocatalysts in the electrolysis cells which absorb the solar energy and convert it to electrochemical energy to split water into oxygen and hydrogen molecules. Among semiconductors, hematite is considered a promising candidate with 16% solar energy conversion efficiency (equivalent to 13 mA/cm2) because of its proper band-gap energy within the visible range of sun-light, abundance in earth’s crust, non-toxicity, and chemical stability. However, it suffers from low hole diffusion depth, short charge carrier lifetime, and low charge separation. We can grow hematite thin films on FTO coated glass substrates by using liquid phase deposition and hyrdrothermal methods. One direction in our group aims in overcoming these obstacles with different strategies such as doping and post-treatment.

1- Andebet Gedamu Tamirat, John Rick, Amare Aregahegn Dubale, Wei-Nien Su and Bing-Joe Hwang, Using hematite for photoelectrochemical water splitting: a review of current progress and challenges, Nanoscale Horizons., 2016, 1, 243-267.
2- M. Saeidi, A. Yourdkhani, Seyed Ali Seyed Ebrahimi, Reza Poursalehi, Candle flame-treatment as an effective strategy to enhance the photoelectrochemical properties of Ti-doped hematite thin films, Journal of Materials Chemistry C, 2020, 8, 11950-11961.
3- Mahdi Rasouli, Amin Yourdkhani, Reza Poursalehi, Photoelectrochemical properties of gradient Ti-doped hematite thin films prepared by liquid phase deposition, Materials Science in Semiconductor Processing, 142 (2022) 106476.
4- Reza Montahaei, S.A. Seyyed Ebrahimi, Amin Yourdkhani, Reza Poursalehi, Photoelectrochemical properties of butane flame-treated niobium-doped hematite thin films grown by the liquid-phase deposition method, Journal of Alloys and Compounds, 894 (2022) 162428.
